Short Squeeze Alert (NASDAQ:SCLX)
With a near HOD of close yesterday our focus is still on (NASDAQ:SCLX)
Good morning traders, we have had a crazy week and it's only wednesday!!
2 Back to back winners but the story of SCLX may just be getting started. The stock started to rally yesterday into the power and closed at $1.05!!!
For most of the opening bell and first few hours of the market being open *SCLX* was trading between $.96 and $.98 .. and then the end of day rally started to happen..
IS this the massive short position starting to cover?
If that's the sound of a squeeze that we hear then today is going to be THE DAY!!!
Ortex.com has the cost to borrow on this at over 140%!!
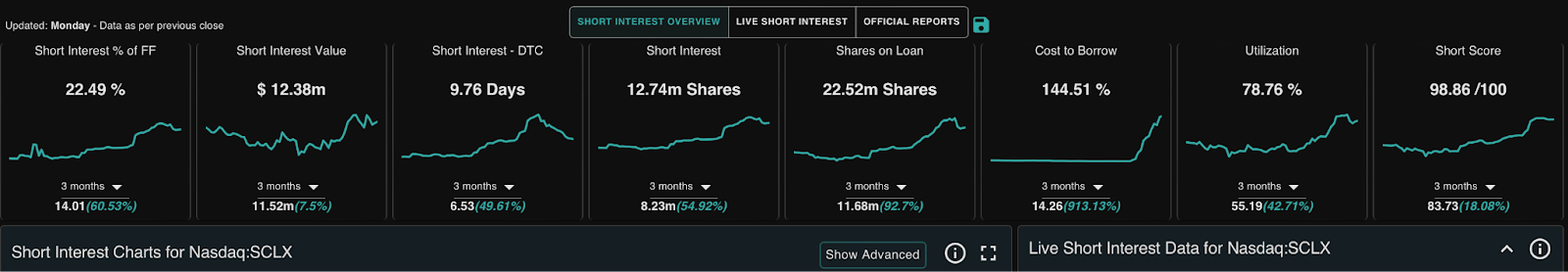
And there are 22 Million shares SHORT!!!!
Are we about to witness this little beauty head into BEAST MODE????
Here we go traders!!
(NASDAQ:SCLX) To The MOON!!
Full report below
A Heavy Short Interest Could Lead to a Monstrous Short Squeeze for this Tiny NASDAQ Healthcare Firm!

As the Opioid crisis continues to claim lives, Scilex Holding Company (NASDAQ: SCLX) is addressing the problem with promising solutions!
The opioid epidemic is the most important and most serious public health crisis today. The effects are reported in overdose deaths but are also starkly evident in declines in sense of well-being and general health.
As per most recent research, JEC analysis has found that the opioid epidemic has cost the U.S. nearly $1.5 trillion in 2020, and this figure is likely increasing each year! The problem is so dire that Congress has approved $10.6 billion in discretionary spending to combat the epidemic.
What is SCLX doing?
Scilex Holding Company (NASDAQ: SCLX) is a revenue-generating company that is focused on addressing one of the biggest issues facing humanity today - the overuse of opioids. The company is focused on developing non-opioid pain management products that provide the relief patients so desperately need, while avoiding the debilitating addiction that often comes with the use of opioids. 
Roughly 112,000 Americans died in 2023 from overdoses, and it is probable that a good number of those involved opioid use at some point to reduce physical pain of various types.
Why pay attention to SCLX right now?
- SCLX already has THREE commercialized products that are already FDA APPOVED and proven to improve patients' lives. The company is filling a much-needed area of the health care sector, that of developing non-opioid pain relief products.
- SCLX has been heavily shorted. Based off of Ortex.com, as of November 1st, 2024, the short interest for SCLX on the Nasdaq was at $12.2M in value with 12.71M shares shorted. There are 22.35M shares on loan.
- A short squeeze could be imminent!
- What is a short squeeze and why does it matter? This is a situation in which the price of a stock rises to such an extent that investors who have sold short, have to quickly purchase the stock in order to limit their losses.
- Insiders are loading up! When a single insider buys stock, it is usually not a big deal. However, when several insiders are buying, like in the case of Scilex Holding Company (NASDAQ:SCLX), it sends a message; they think the stock will go up!
- Over the last quarter, Scilex Holding insiders have spent a meaningful amount on shares. They collectively bought US$94k worth of shares.
- In the last twelve months Scilex Holding insiders were buying shares and NOT selling!
- Zacks Small Cap Research is BULLish on the company.
- The research firm believes that "SCLX is poised to resume its move higher and that investors continue to underestimate the value of the treatments the company currently has and is developing."
- Zacks SCR has a $5.50 price target as seen HERE.
- SCLX announced a financing deal that helps to pay off debt and adds cash at good terms!
- The deal bolsters the company's financial position and positions SCLX for future growth.
- Under the deal, Scilex is issuing convertible notes and issuing 7.5 million warrants. As a result, the company is going to net approximately $20.5 million. Management notes that it intends to use these proceeds to satisfy $12.5 million of a note and pay off another revolving credit facility.
- The company is refinancing debt at better terms and potentially providing cash at good terms for future capital needs.
Keep on reading to discover why SCLX should be high on your radar!
The company's exciting narrative could fuel tremendous growth ahead and may lead to short investors having to cover their positions FAST!

Overview
SCLX is committed to developing and commercializing innovative therapies that address significant unmet medical needs in chronic pain management.

Company Features

Partners Include

A Leader in Non-Opioid Pain Management Products
Scilex Holding Company (Nasdaq: SCLX) is an innovative revenue-generating company focused on acquiring, developing and commercializing non-opioid pain management products for the treatment of acute and chronic pain and becoming the global pain management leader committed to social, environmental, economic, and ethical principles to responsibly develop pharmaceutical products that maximize quality of life.
Scilex Pharmaceuticals and Semnur Pharmaceuticals are wholly owned subsidiaries.
Three Commercialized Products
Scilex Pharmaceuticals, Inc. has three FDA-approved commercial products on the market and 3X version follow-on product, SP-103, is the next generation of ZTlido®:
The company's lead product ZTlido® (lidocaine topical system) 1.8%, is a marketed prescription lidocaine topical product approved by the U.S. Food and Drug Administration for the relief of pain associated with Post-Herpetic Neuralgia (PHN), which is a form of post-shingles nerve pain. ZTlido® possesses novel delivery and adhesion technology designed to address many of the limitations of current prescription lidocaine patches by providing significantly improved adhesion and continuous pain relief.
The company acquired two FDA approved non-opioid pain products, GLOPERBA® and ELYXYB®. GLOPERBA® is indicated for the prophylaxis of gout flares in adults. Elyxyb is indicated for the acute treatment of migraine with or without aura in adults. We launched ELYXYB® in the U.S. in April 2023 , GLOPERBA® was launched on June 10th, 2024.
- ZTlido® (lidocaine topical system) 1.8%, a prescription lidocaine topical product for the relief of neuropathic pain associated with postherpetic neuralgia, which is a form of post-shingles nerve pain with an average of 50% growth in gross sales for the past two years. ZTlido® is expected to be distributed outside of the U.S. in 2025 with exclusive territory distributors in the Middle East and North/South Africa countries with a $105 million minimum 5 year purchase commitment.
- ELYXYB® is a first-line treatment and the only FDA-approved, ready-to-use oral solution for the acute treatment of migraine, with or without aura, in adults.1 The U.S. oral migraine drug market size was estimated to be $1.8 billion in 2022.2 ELYXYB® filed a New Drug Submission (NDS) to Health Canada's Pharmaceutical Drugs Directorate, Bureau of Cardiology, Allergy and Neurological Sciences for the approval of for acute treatment of migraine with or without aura in Canada. It is estimated to have impacted more than 2.7 million Canadians with the Canadian migraine therapeutics market estimated to reach approximately $400 million by 2025.3
- Gloperba®, the first and only liquid oral version of the anti-gout medicine colchicine indicated for the prophylaxis of painful gout flares in adults. Gout is a painful arthritic disorder affecting an estimated 9.2 million people in the United States4. The gout treatment market is projected to reach $2.0 billion in the U.S. by 2028 with a well-defined area of unmet need.5
- Source: Celecoxib Oral Solution Approved for Acute Migraine March 2020.
https://www.neurologylive.com/view/celecoxib-oral-solution-gets-goahead-for-acute-migraine - Source: Evaluate Pharma data February 16, 2023
- Source: Mordor Intelligence - MIGRAINE THERAPEUTICS MARKET (2020-2025)
- https://jamanetwork.com/journals/jama/fullarticle/2787544#:~:text=How Common Is Gout?,% of the adult population
- Evaluate Pharma data
ZTlido Performance
ZTlido is the #1 prescribed branded non-opioid analgesic by the pain specialist! Over 1MM patients have been treated with ZTlido as of last year. According to market research, patient satisfaction with ZTlido was ~90%.
ZTlido Q2-2024 Sales Performance –
- ZTlido net sales for the quarter ended June 30, 2024 were $14.5 million, compared to $12.2 million for the same period last year, representing growth of approximately 19%.
- Total product net sales for the quarter ended June 30, 2024 were $16.4 million, compared to $12.6 million for the same period last year, representing growth of approximately 30%.
Based on the independent market research conducted by Syneos Health Consulting, with the new campaign, health care providers (HCPs) report increased awareness and substantial intent to utilize for ZTlido® with peak sales potential projected to reach over $500 million in the next 6 years in the U.S.!
Target Patients for Gloperba Today
- Patients with CKD Stage 3/4/5: 6 million patients
- Patients with GI tolerability issues: 1 million patients
- Patients who have difficulty swallowing

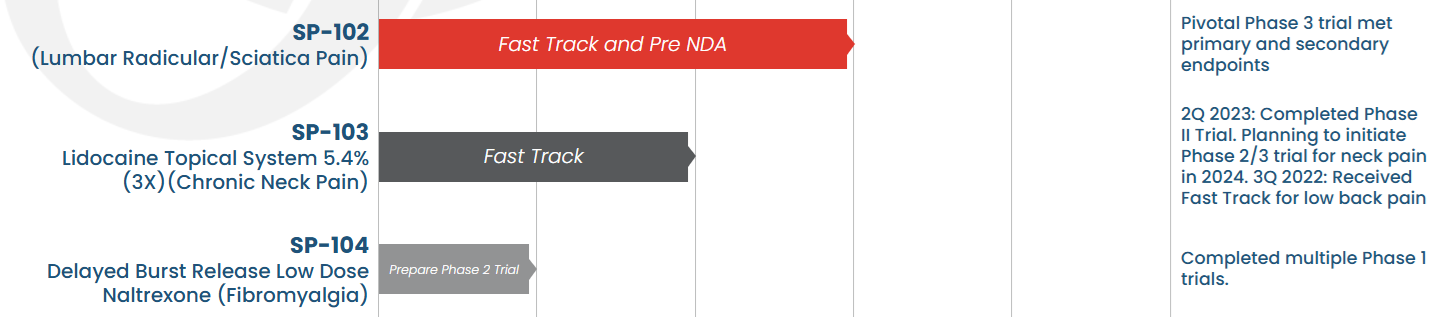
Three Product Candidates
- SP-102 (10 mg, dexamethasone sodium phosphate viscous gel) ("SEMDEXA™"), a novel, viscous gel formulation of a widely used corticosteroid for epidural injections to treat lumbosacral radicular pain, or sciatica, has received Fast Track Status from the FDA. C.L.E.A.R Trial, a Phase 3 study of SP-102 has been completed and met its primary and secondary endpoints. Currently there is no FDA approved non-opioid epidural injection to treat lumbosacral radicular pain, or sciatica; SP-102 (SEMDEXA ) is On-Track to be the First Product Approved to Treat Sciatica!!
- SP-103 (lidocaine topical system) 5.4%, ("SP-103"), a next-generation, triple-strength formulation of ZTlido®, has recently completed a Phase 2 trial in acute lower back pain ; planning is underway for a Phase 2/3 trial in chronic neck pain.
- It is estimated that the U.S. low back and neck pain market will reach $134.5 billion.6 Based on the independent market research conducted by Syneos Health Consulting ("Syneos"), with the substantial intent in utilization for SP-103 with peak sales potential projected to reach $1.2 billion annually in the 6th year post launch.
- SP-104 (4.5 mg, low-dose naltrexone hydrochloride delayed-release capsules) ("SP-104"), is a novel low-dose delayed-release naltrexone hydrochloride being developed for the treatment of fibromyalgia.
The company believes that its products and product candidates have the potential to provide effective non-opioid prescription pain medication options for patients and healthcare providers who seek alternatives to opioids and NSAIDs for various pain conditions.
If these product candidates are approved by the FDA, SCLX believes each of them could become the treatment option for their respective indications in the United States!
FDA approval may come sooner than later for SP-103…
There has been a successful end of Phase II meeting with the FDA leading to an agreed path forward to NDA upon completion of Phase III trials for the blockbuster product candidate, SP-103, for the treatment of chronic neck pain associated with muscle spasms!
"We are very pleased with the end of Phase II meeting and received a clear path forward to NDA for our blockbuster product candidate, SP-103. SP-103 has the potential to meet our core goal of developing leading pain management therapies to significantly improve the lives of patients for the treatment of chronic neck pain associated with muscle spasms who are seeking new effective treatments. We are looking forward to conducting Phase 3 trials and believe that Scilex is the only company with technology allowing much higher lidocaine concentration than any other topical lidocaine system treatments. Higher concentration of a drug per covered area of skin is important for achieving therapeutic response." - Dmitri Lissin, M.D., Chief Medical Officer of Scilex.
Leadership
The mission of SCLX's management team is to bolster our proprietary pain management clinical pipeline and technology by employing years of expertise that come with Pain Management Products, Clinical Pipeline, and technology. These seasoned professionals bring a type of leadership that is unmatched in the pharmaceutical industry!
Jaisim Shah - Chief Executive Officer & President
Jaisim Shah has over 30 years industry success in leading product development & commercializing innovative therapies and creating companies with documented success in development and commercialization of some of today's most recognized pharmaceutical brands.
Henry Ji, PhD - Executive Chairman
Henry Ji brings 25+ years of experience in the biotechnology and life sciences industry. Dr. Ji has been Chairman of Scilex from March 2019 to present and served as the CEO of Scilex Pharmaceuticals from November 2016 to March 2019.
Suketu D. Desai, PhD - Chief Technical Officer & Senior Vice President
Suketu D. Desai, Ph.D. has 25+ years of experience in the Biologics and Pharmaceutical Industry. Suketu is Chief Technical Officer and Senior Vice President, Chemistry, Manufacturing and Controls, Regulatory CMC, and Quality Assurance at Scilex Pharmaceuticals (2015 – present).
Dmitri Lissin, MD - Chief Medical Officer & Senior Vice President
Dmitri Lissin currently serves as Chief Medical Officer and Senior Vice President, Clinical Development and Medical Affairs of Scilex/Semnur Pharmaceuticals (2015 – present).
Suresh Khemani - Chief Commercial Officer & Senior Vice President
Mr. Khemani brings more than 25 years of global pharmaceutical experience to Scilex, a privately held clinical development and commercial company based in Palo Alto, California.
Steve Lincoln - General Counsel & Chief Compliance Officer
Steve Lincoln serves as General Counsel, Chief Compliance Officer for Scilex Holding Company. Steve has been involved in the biopharma industry for more than 21 years.
Stephen Ma - Chief Financial Officer & Senior Vice President
Stephen Ma has served as the Company's Chief Accounting Officer since November 2022 and previously served as its Vice President of Finance from January 2022 to November 2022.
Elaine K Chan, PharmD - Executive Director and Head of Medical Affairs
Dr. Elaine Chan heads Medical Affairs at Scilex, since 2022. Prior to joining Scilex, Dr. Chan was Senior Director at Genentech Inc (a member of the Roche group), one of the world's leading pharmaceutical companies, from 2009 to 2021, where she held wide-ranging roles in Medical Affairs, Business Operations and Strategy, Pharmacovigilance, Marketing and new product planning.
Gigi DeGuzman - Senior Executive Director and Chief of Staff
Gigi DeGuzman is a seasoned professional with over 25 years of experience in human resources, operations management, facilities management, event planning, executive coordination, and administrative operations.
Mike Ciaffi - National Sales Director
Mike has over 25 years in the pharmaceutical industry. Mike currently serves as the National Sales Director at Scilex Holding Company. Mike joined Scilex in 2018 as a Regional Business Director to build a sales team to launch ZTLido (Lidocaine Topical System) 1.8%.
Sumant Rajendran - Executive Director of Marketing
Sumant Rajendran heads up the Marketing department and brings over 20 years' experience to Scilex. He has been at the company since mid-2019 and has overseen the growth of our marketed portfolio from one product (ZTlido) to three (with the addition of Elyxyb and Gloperba).
In Summary
Scilex Holding Company (NASDAQ: SCLX) is a small cap NASDAQ firm that is an innovative leader in non-opioid pain therapeutics. The company's offerings could significantly aid in stopping the opioid crisis and create maximum shareholder growth!
To reiterate the company's investment highlights:
- Tremendous Insider Ownership
- 3 FDA approved Non-Opioid Acute and Chronic Pain Management Products
- Worldwide Commercial Rights to Most Product Candidates
- Strong Proprietary Platform with High Barriers to Entry
- Established Reimbursement Access
- Blockbuster Pipeline with Limited Capital Required for Commercialization
Not long ago SCLX also announced the closing of a financing deal that may bolster the company's financial position and position the company for future growth.
With a substantial short position on SCLX currently, there could be a massive short squeeze on the horizon.
Short squeeze has become a buzzword in the investment world after the skyrocketing of GameStop Corp. and AMC Entertainment Holdings Inc. stock hit global media headlines.
The short squeeze of AMC Entertainment Holdings Inc. (NYSE: AMC) stock occurred in 2021. According to S3 Partners, about 20% of all the US cinema chain's shares were traded in short positions at that time. Note that on average this figure is equal to 5%. This large volume of selling positions triggered a short squeeze: on 4 January 2021, AMC Entertainment stock traded at the price of 2.01 USD per share; while during the trading session on 2 June, it reached 72.62 USD. The share price skyrocketed 3512.94% in half a year.
SCLX is a short squeeze stock that could take off so put it high on your watch list!